June 1st, 2022- Brisbane, Australia- Australian life science company, QBiotics will showcase positive efficacy data from its completed phase I trial of lead cancer molecule, tigilanol tiglate, and its Phase II clinical program at Bio 2022, the world’s largest gathering of biotechnology and pharma leaders.
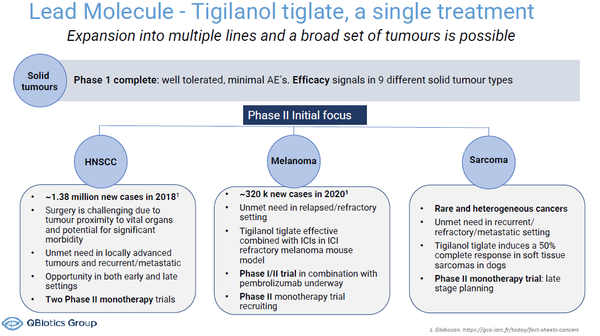
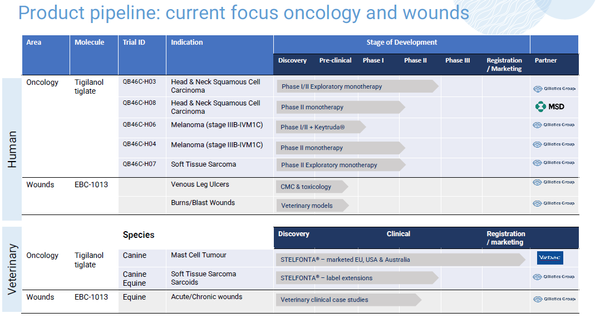
Tigilanol tiglate is a novel small molecule that has “agnostic activity” and in development as an intratumoral treatment for a range of solid tumors, initially Head and Neck Squamous Cell Carcinoma (HNSCC), melanoma and soft tissue sarcoma.
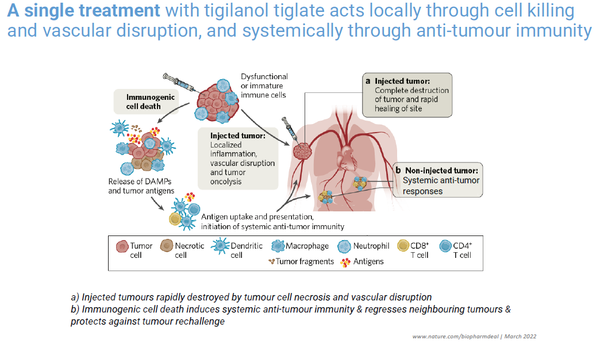
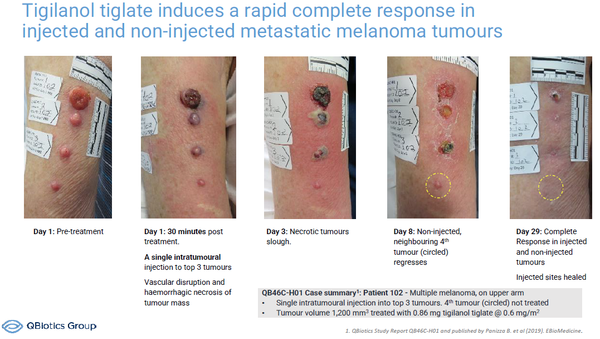
With a single intratumoral injection, tigilanol tiglate acts directly to destroy tumors through tumor cell oncolysis and vascular disruption and indirectly through systemic anti-tumor immunity.
In 2020, there were 19.3 million newly diagnosed cases of cancer globally(1), with solid tumors accounting for 90% of all cancers(2). Meanwhile, HNSCC is the 7th most common cancer globally, with around 1.38 million new cases reported in 2018(1). There were 0.32 million new cases of melanoma(1).
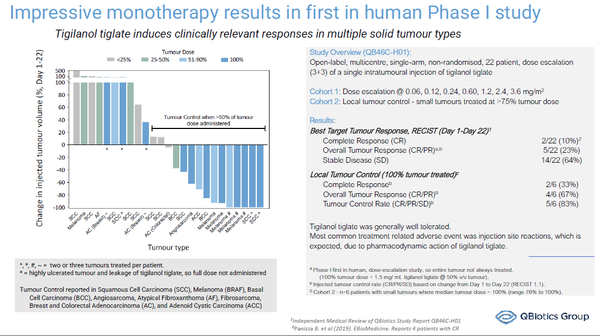
At Bio 2022 QBiotics will invite discussions with potential investors, partners and collaborators about the positive data from the company’s completed phase I study of tigilanol tiglate in solid tumors(3), as well as its ongoing and planned Phase II clinical trials in patients with HNSCC, melanoma and soft tissue sarcoma.
While effective as a single agent, QBiotics will also discuss encouraging pre-clinical results of tigilanol tiglate inducing synergistic anti-tumor responses when combined with other cancer therapies, including chemotherapy, radiotherapy and checkpoint inhibitors. QBiotics is pursuing monotherapy as well as combination therapy development paths, with a trial in patients with melanoma with MSD’s (Merck & Co. Inc., Kenilworth, USA) immune checkpoint inhibitor, Keytruda®.
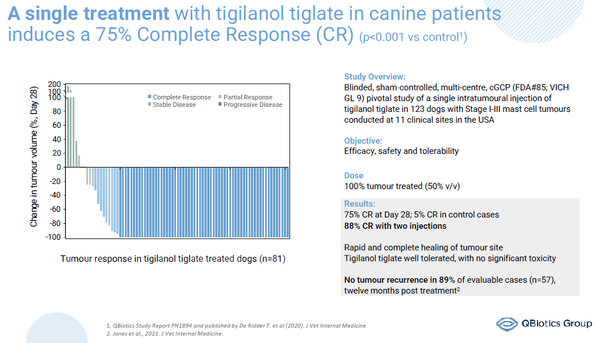
In support of QBiotics’ global development plan is a strong foundation of support with its launch of STELFONTA® (tigilanol tiglate) for the treatment of canine mast cell tumours in the USA, Europe and Australia through Virbac, a global animal health company. With a single injection, STELFONTA® (tigilanol tiglate) induces a 75% complete response in dogs(4) and provides a first-line alternative to surgery in non-metastatic mast-cell tumors, meeting an unmet need in the veterinary market providing QBiotics with an income stream, powering the company for future growth.
QBiotics will be meeting with companies to foster new partnerships to assist in the acceleration and expansion of tigilanol tiglate for multiple oncology indications.
- Globocan 2020.https://gco.iarc.fr/today/fact-sheets-cancers
- National Cancer Institute. Surveillance, Epidemiology, and End Results Program (SEER) https://training.seer.cancer.gov/disease/categories/classification.html
- Panizza et al., 2019. eBioMedicine 50. 433-441. doi.org/10.1016/j.ebiom.2019.11.037
- De Ridder et al., 2020. J Vet Intern Med. 1-15. DOI: 10.1111/jvim.15806